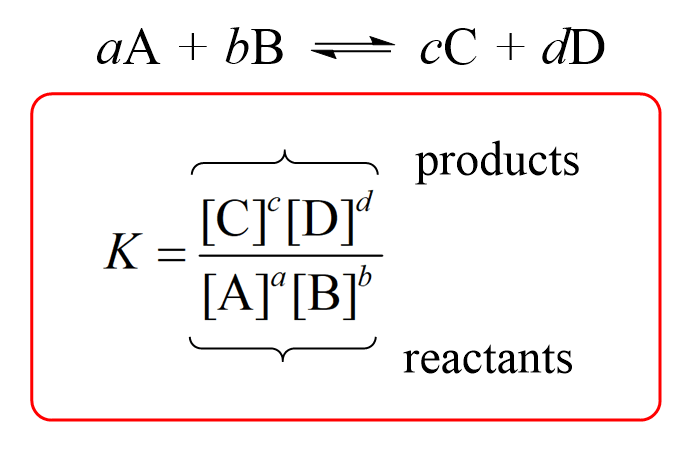
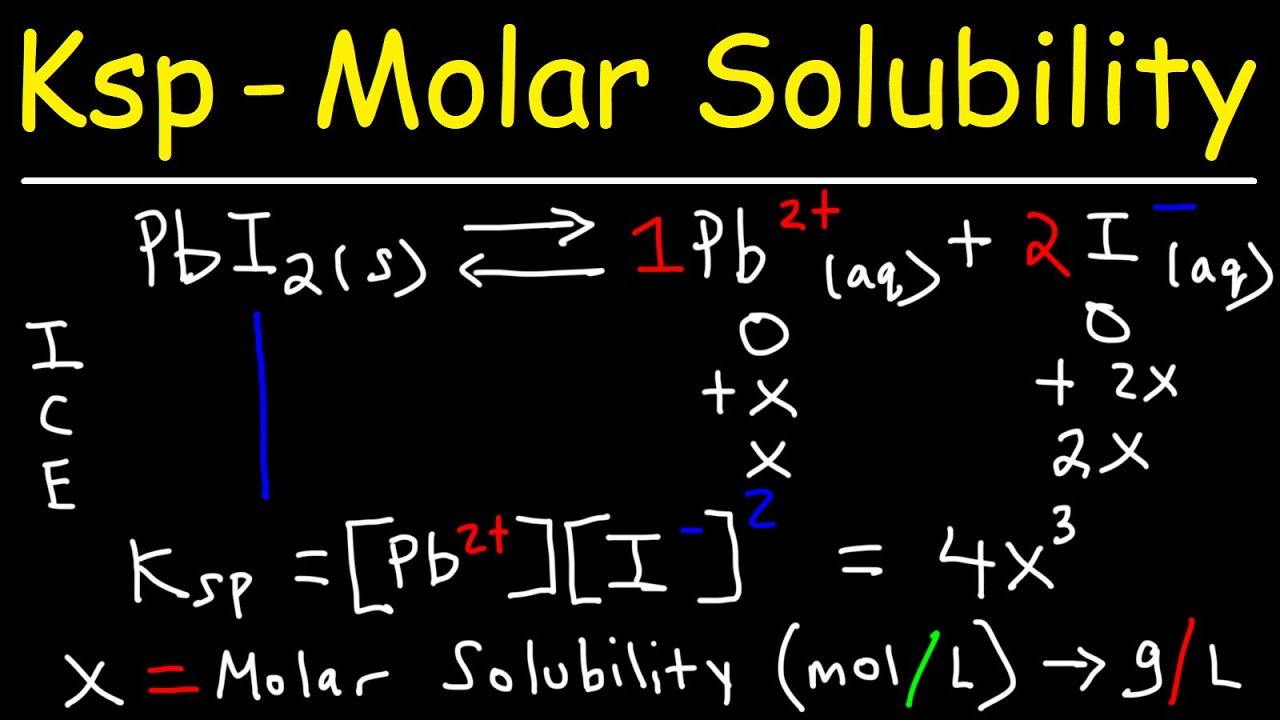
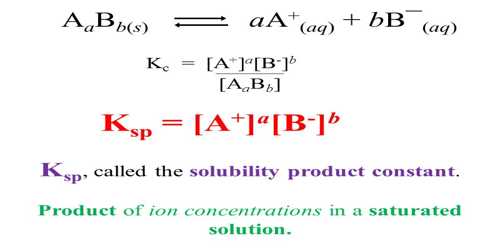
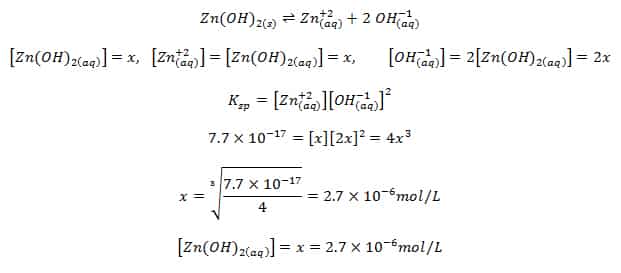![Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download](https://images.slideplayer.com/27/9060864/slides/slide_3.jpg)
Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download

The calculated values of solubility parameters of polymers and solvents. | Download Scientific Diagram
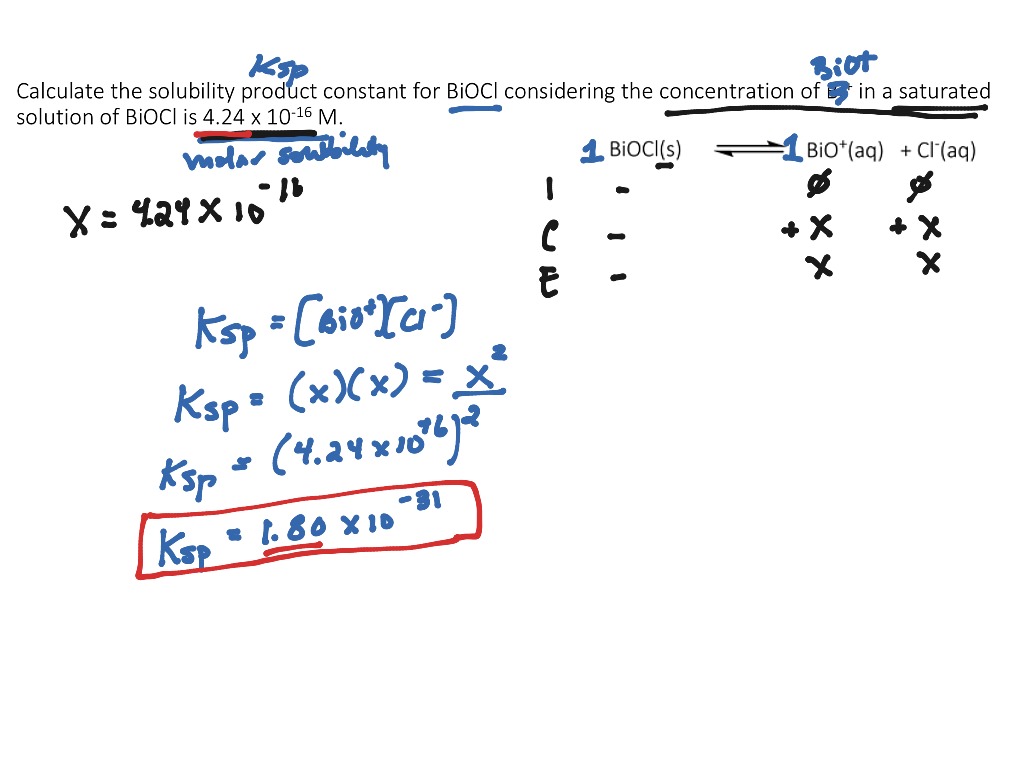
Calculating the Solubility Product Constant (Ksp) for an Insoluble Salt | Science, Chemistry | ShowMe
calculate solubility of silver benzoate in a buffer solution of ph=3.19 the ionisation cons†an t of benzoic acid is 6.46∗10^ 5 and Ksp for silver benzoate is 2.5∗10^ 13 (b) also calculate
Calculate the solubility product and solubility of AgCl in the following cell which has an emf of 0.455 volts at 25°C. - Sarthaks eConnect | Largest Online Education Community