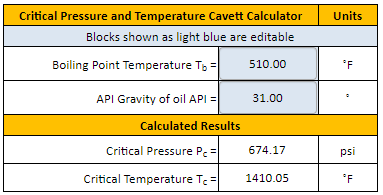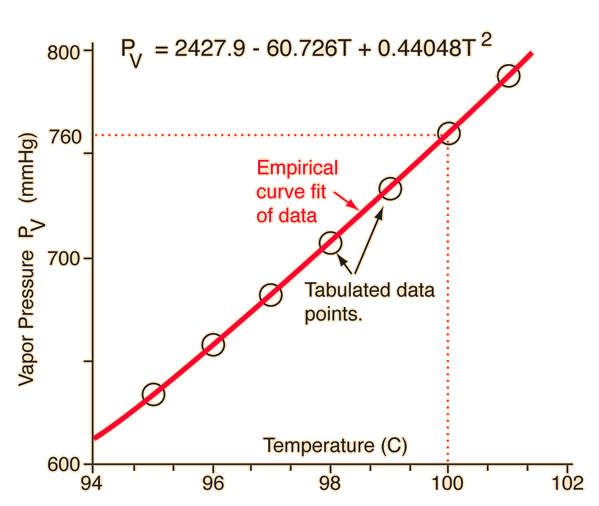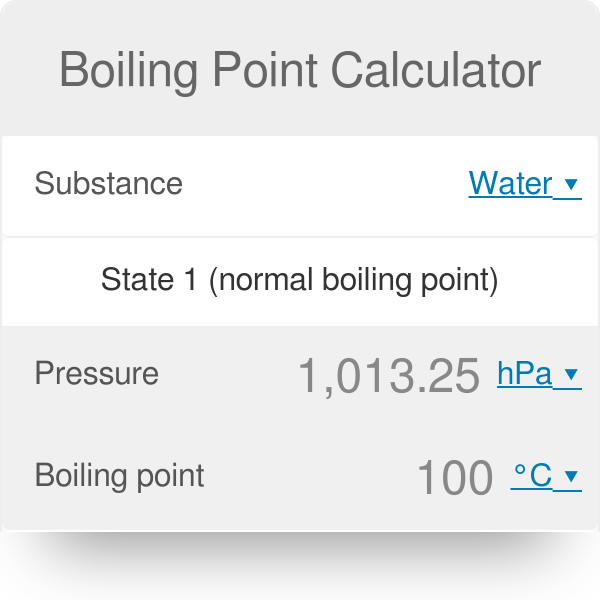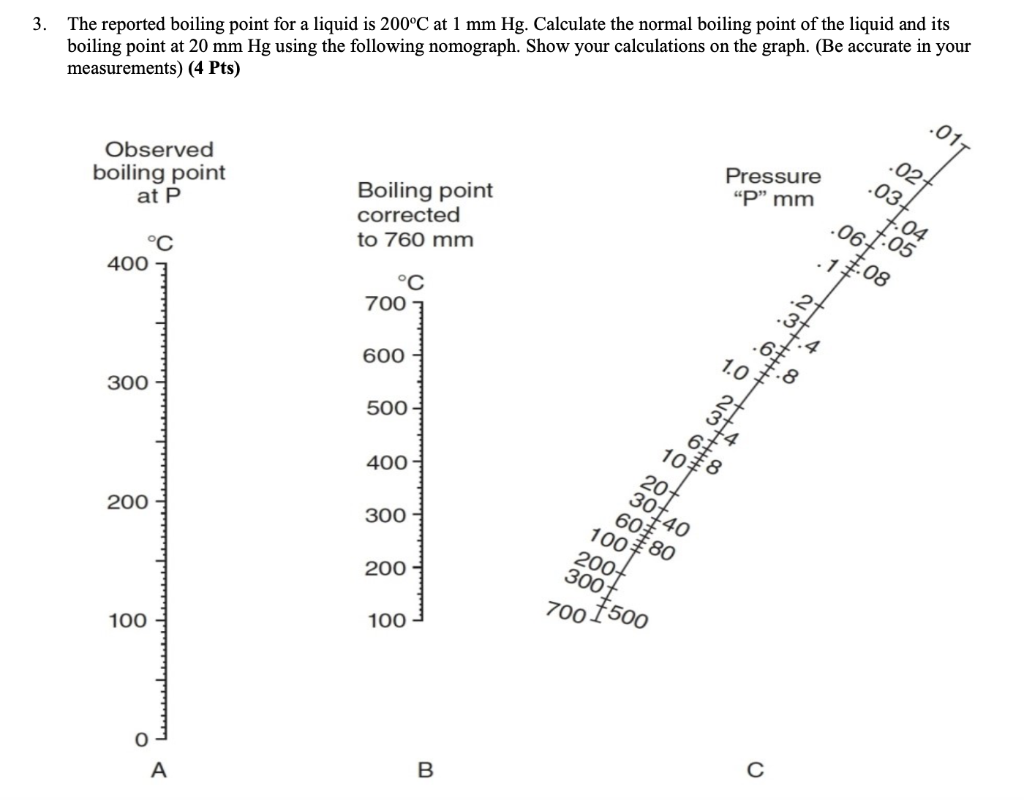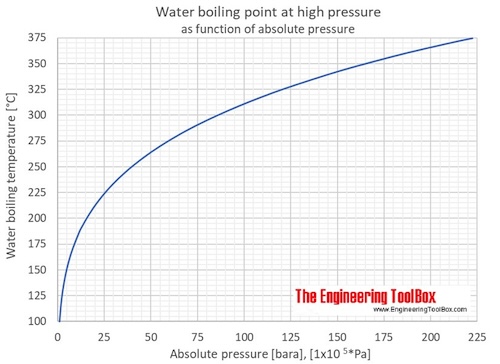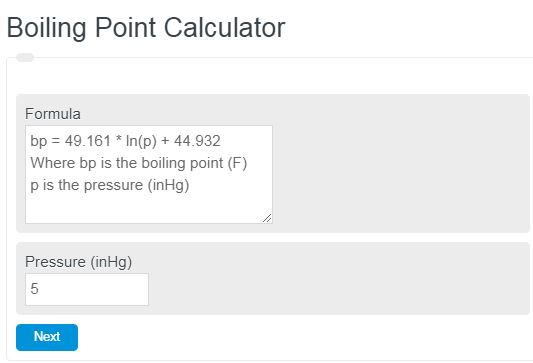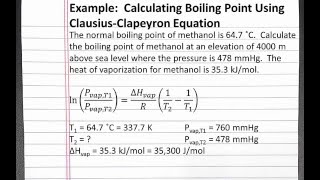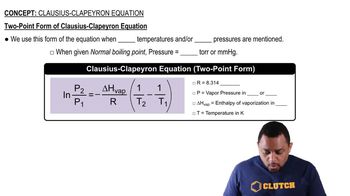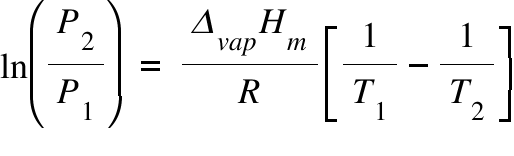
Correlation of Vapor Pressure at Different Temperatures by Clausius Clapeyron Equation Calculator | Calistry

Methanethiol has a vapor pressure of 429 torr at −25 ∘c and a normal boiling point of 6.0 ∘c. find δhvap - Brainly.com
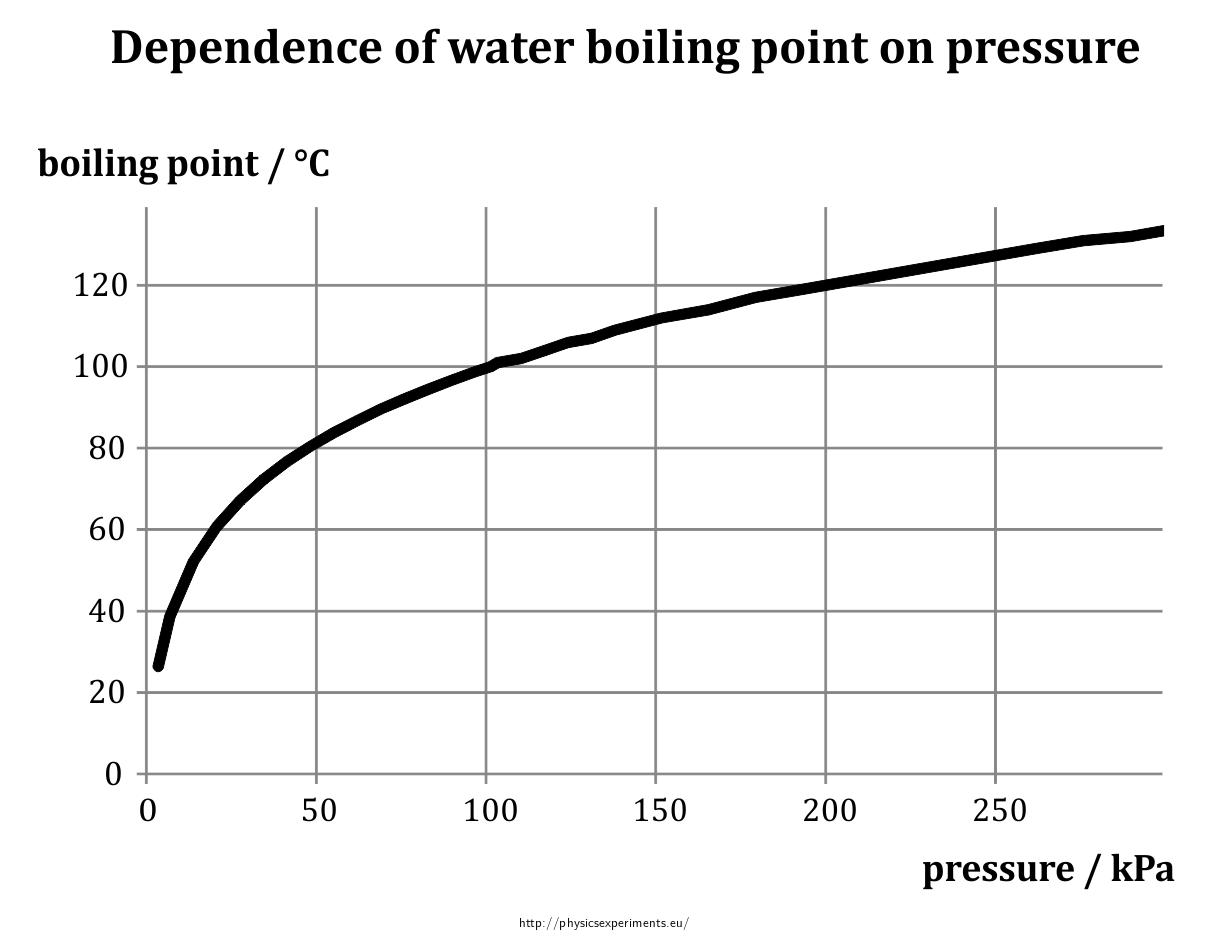
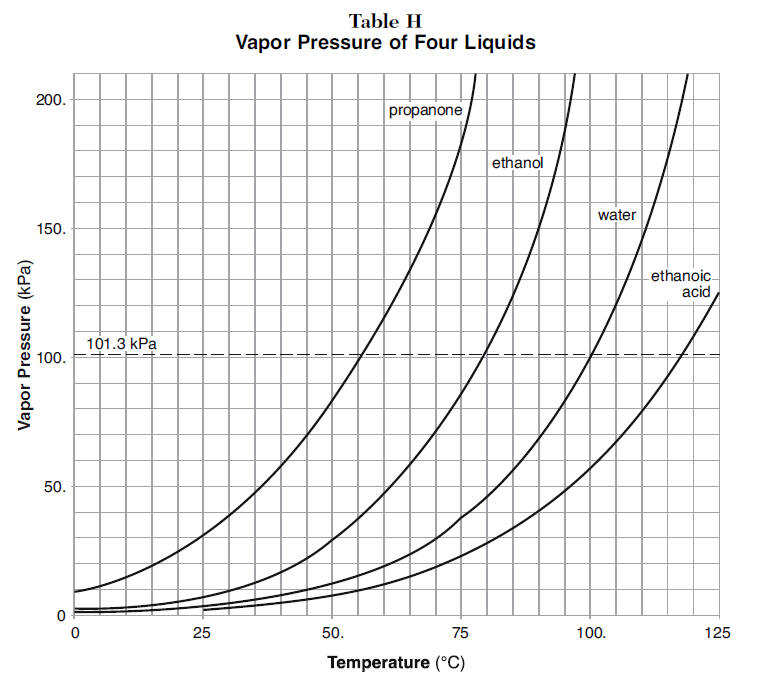
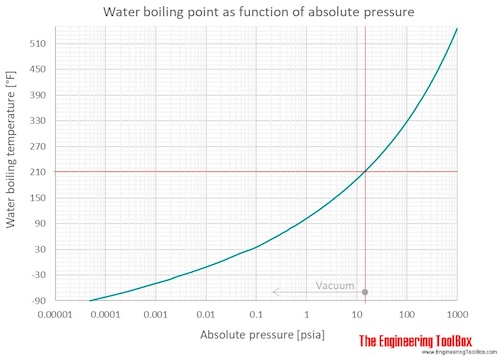
How does one calculate the boiling point of water at different pressures without a steam table? - Quora

The vapour pressure of a pure liquid at 25 is 100 mm Hg . Calculate the relative lowering of vapour pressure if the mole fraction of solvent in solution is 0.8.
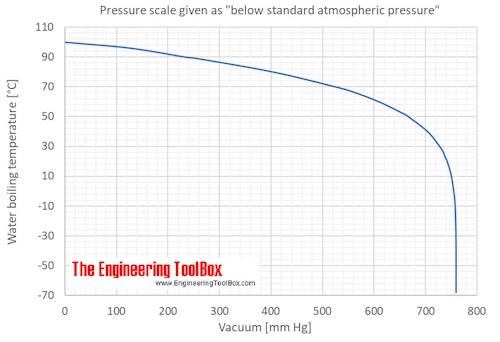
What is the boiling point of water at a base camp of Mount Everest if the atmospheric pressure is 224mmHg? - Quora